Lipid Research
Melitherapy (short for Membrane-Lipid Therapy) is an innovative scientific platform based on the discovery and rational design of drugs which specifically target lipids of the cell membrane. This contrasts with the usual approach to drug design, which targets proteins to elucidate their structure and then uses molecules (chemicals, antibodies, etc.) that directly regulate their activity.
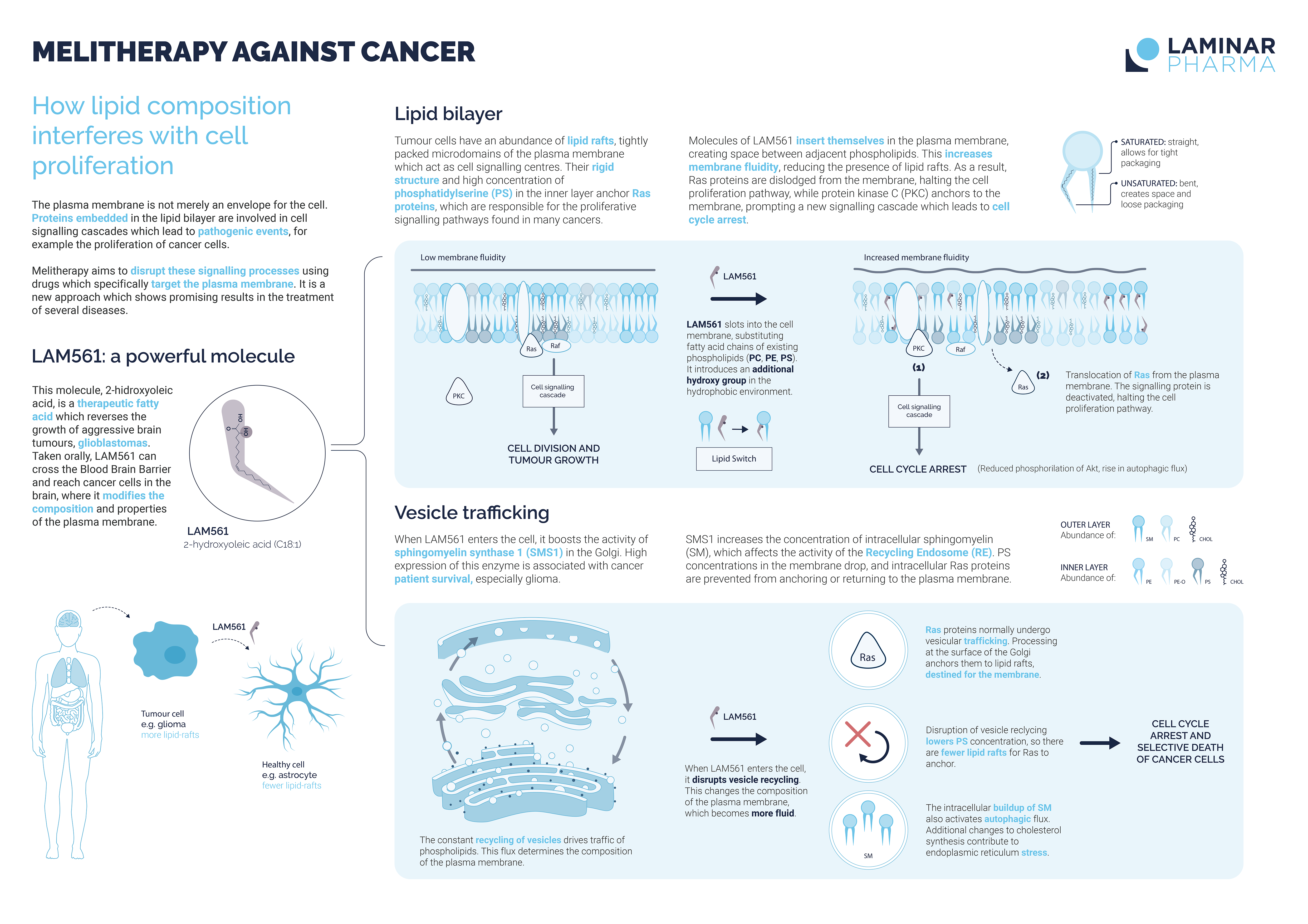
In melitherapy, we use synthetic fatty acids that modify the composition and fluidity of the cell membrane to interfere with the activity of membrane-associated proteins responsible for disease processes, such as tumor growth.
Thanks to this disruptive innovation, we will develop new medicines with an exceptional safety/efficacy combination for serious pathologies where other treatments fail, such as cancer, neurodegenerative or metabolic diseases.
Through different clinical trials, we are evaluating the potential translation of significant clinical benefit to patients who receive melitherapy drugs. Study MIN-001-1203 is the first clinical trial with 2-hydroxyoleic (LAM561A1) in patients with advanced solid tumors, including malignant glioma, and it has already been completed with very promising results.
Our most advanced line of research is for therapies in oncology. Several of our late-stage clinical trials focus on the very promising antitumoral effects of 2-hydroxyoleic acid (LAM561A1) in glioma, brain tumor, in adults and in children.
Since our origins in 2006, we have focused on rare, pediatric and geriatric diseases, especially those with unmet medical needs. We are also developing melitherapy drugs for Alzheimer’s disease, and for metabolic, cardiovascular and inflammatory diseases.

A Phase 1/2a Dose Escalation Study of 2-Hydroxyoleic Acid (2-OHOA) in Adult Patients with Advanced Solid Tumours Including Malignant Glioma.
EudraCT #2012-001527-13 – clinicaltrials.gov #NCT01792310
This trial is an open label, non-randomized study in patients with advanced solid tumours including malignant glioma and consists of two phases: (1) a dose-escalation phase following a standard “3+3” design to establish dose-limiting toxicity (DLT) and a safe dose of 2-OHOA and (2) an expansion phase with two expanded safety cohorts (approximately 10 of whom have malignant glioma and approximately 10 of whom have other advanced solid tumours that are suitable for biopsy) treated at the maximum tolerated dose (MTD). MIN-001-1203 trial has been conducted in leading investigational sites in London, Newcastle, Barcelona, San Sebastian and Bilbao.
Recruitment and treatment of patients in MIN-001-1203 study have completed. A total of 54 patients have been treated, of which 46 are evaluable for safety assessment. 32 patients were recruited in 7 cohorts in the Dose-Escalation Phase and 22 patients in two safety expansion cohorts in the expanded phase. Results of the study are currently being evaluated and have confirmed an excellent safety profile, while promising clinical activity (by RANO / RECIST v1.1) has been reported in several cases, including patients with recurrent malignant glioma.
A Phase 1b Dose Finding Study of the Safety of 2-Hydroxyoleic Acid Sodium Salt (2-OHOA) Administered Orally in Combination with Temozolomide and Radiation Therapy (Concurrent Phase) or Temozolomide Alone (Maintenance Phase) in the First Line Treatment of Subjects with Glioblastoma.
EudraCT #2018-000317-21 – clinicaltrials.gov #NCT03867123
First-line treatment for patients with GBM consists of a concurrent phase (one 6-week cycle with daily administration of temozolomide (TMZ)) during which TMZ is given with Radiation Therapy (RT), followed by a rest phase (4 weeks in duration;), and a maintenance phase, during which patients receive TMZ for up to six 28-day cycles. The purpose of this study is to determine the highest safe dose of 2-OHOA administered orally in combination with temozolomide (TMZ) and radiation therapy (RT) during the concurrent phase, and in combination with TMZ alone during the maintenance phase. This study has been successfully executed in 3 hospitals in Spain without any adverse event due to 2-OHOA treatment.
A Phase 1/2a Study of 2-Hydroxyoleic Acid in Pediatric Patients with Malignant Glioma and Other Advanced Solid Tumors.
clinicaltrials.gov: #NCT04299191
An open label, non-randomized study in pediatric patients with advanced high-grade gliomas and other solid tumors. The study will be performed in two phases – a dose escalation phase in up to 18 patients following a standard “3+3” design to establish dose limiting toxicity (DLT) and a “safe” dose of 2-OHOA followed by an expanded safety cohort of up to 10 patients treated at the MTD. If the MTD is well tolerated in the expanded safety cohort, that dose becomes the RP2D.
IND approved. Patient recruitment opened in the USA leaded by the pediatric clinical research institution Hackensack UMC, NJ.
A Phase 2b/3, Randomized, Double-Blind, Adaptive, Placebo-Controlled Adjuvant Trial in Newly Diagnosed Glioblastoma (NDGBM) Patients to Assess the Efficacy and Safety of 2-Hydroxyoleic Acid (LAM561) in Combination with Radiotherapy and Temozolomide Standard of Care Treatment.
EUdraCT # 2018-000365-37 – clinicaltrials.gov: #NCT04250922
Recruitment completed
This is a randomized, double-blind, placebo-controlled, 2 parallel arms (1:1 ratio), adjuvant trial to assess the efficacy and safety of 2-hydroxyoleic acid (LAM561) versus placebo in patients with newly diagnosed GBM IDH wildtype. In both arms, patients will receive the SoC and will be randomized to receive either placebo (Arm A) or 2-OHOA (Arm B).
PIIB Protocol design and regulatory strategy agreed with EMA (Sep. 2018) CTA process ongoing in IL, SP, FR, UK, IT & NL. Up to 25 sites planned to recruit patients as of Q1 2019.
Laminar Pharmaceuticals is a pioneering biopharmaceutical company, focused on the discovery, rational design, and initial clinical development of drugs to treat oncological and other pathologies. All our compounds are based on a novel therapeutic approach: Membrane Lipid Therapy (MLT), or melitherapy for short. We are a science-driven company committed to putting patients first. Our goal is to provide access to our investigational medicines at the appropriate time and in the correct manner for patients.
Laminar Pharmaceuticals understands that some patients may wish to access investigational medicines that are not yet approved by the regulatory authorities. The most appropriate way for patients to access our investigational products is by participating in our clinical trials, which are managed by a team of independent medical and clinical experts and are specifically designed to determine the safety and efficacy of the investigational products so that their clinical benefits and risks can be adequately understood, and to ensure that quality of life improvements or effects compared to existing therapies can be considered against any potential adverse effects. Patients who are interested in participating in one of our studies are encouraged to discuss their specific needs with their physician.
Information regarding our ongoing clinical trials can be accessed at www.clinicaltrials.gov or in our website www.laminarpharma.com/lipid-research_/#clini.
Expanded Access, also called compassionate use or early access, enables some patients with serious or life-threatening diseases without available therapeutic alternatives, who would not otherwise have been eligible to participate in our clinical trials, to gain access to investigational treatment outside of a clinical trial. Expanded access is different from a clinical trial in which the primary purpose is to collect extensive safety and efficacy data to support submission of an application to regulatory agencies to market a drug.
Laminar Pharmaceuticals does recognize and understand there might be patients interested in obtaining access to our investigational products for treatment through Expanded Access but we are unable to manufacture our investigational drugs in the quantities that may be needed for expanded access purposes. Therefore, to ensure availability of investigational material for clinical research and to maintain the safety of potential patients, Laminar Pharma does not currently provide access to our investigational products through Compassionate use/Expanded Access.
This policy may be revised by Laminar Pharmaceuticals at any time. If you have any questions, please reach out to your physician, or contact clinical.dev@laminarpharma.com.
We currently head a consortium of 12 leading clinical and research institutions from across Europe in the CLINGLIO project, designed to test the efficacy and safety of our most promising anticancer drug, LAM561 (2OHOA). Our goal is to execute “A Clinical Phase IIB trial with 2OHOA in patients with newly-diagnosed malignant glioma”, and to this end we have been awarded a 6,15 million euro grant by the European Commission. Find out more at the CLINGLIO project website. We are also working with the Fundación Rioja Salud, in Spain.







2024 – Rodríguez-Lorca R, Román R, Beteta-Göbel R, et al. Targeting the Notch-Furin axis with 2-hydroxyoleic acid: a key mechanism in glioblastoma therapy. Cell Oncol (Dordr). Published online October 14, 2024. doi:10.1007/s13402-024-00995-x
2024 – Escribá PV, Gil-Agudo ÁM, Vidal Samsó J, et al. Randomised, double-blind, placebo-controlled, parallel-group, multicentric, phase IIA clinical trial for evaluating the safety, tolerability, and therapeutic efficacy of daily oral administration of NFX88 to treat neuropathic pain in individuals with spinal cord injury. Spinal Cord. 2024;62(8):454-467. doi:10.1038/s41393-024-01006-4
2024 – Kakhlon O, Saada A, Escriba PV. Editorial: Metabolic modulation of cellular function [published correction appears in Front Cell Dev Biol. 2024 Apr 11;12:1403128. doi: 10.3389/fcell.2024.1403128]. Front Cell Dev Biol. 2024;12:1395922. Published 2024 Mar 18. doi:10.3389/fcell.2024.1395922
2023 – Agrillo B, Porritiello A, Gratino L, et al. Antimicrobial activity, membrane interaction and structural features of short arginine-rich antimicrobial peptides. Front Microbiol. 2023;14:1244325. Published 2023 Oct 5. doi:10.3389/fmicb.2023.1244325
2023 – Lopez J, Lai-Kwon J, Molife R, et al. A Phase 1/2A trial of idroxioleic acid: first-in-class sphingolipid regulator and glioma cell autophagy inducer with antitumor activity in refractory glioma. Br J Cancer. 2023;129(5):811-818. doi:10.1038/s41416-023-02356-1
2023 – Fernández-García P, Malet-Engra G, Torres M, et al. Evolving Diagnostic and Treatment Strategies for Pediatric CNS Tumors: The Impact of Lipid Metabolism. Biomedicines. 2023;11(5):1365. Published 2023 May 5. doi:10.3390/biomedicines11051365
2023 – Álvarez R, Escribá PV. Structural Basis of the Interaction of the G Proteins, Gαi1, Gβ1γ2 and Gαi1β1γ2, with Membrane Microdomains and Their Relationship to Cell Localization and Activity. Biomedicines. 2023;11(2):557. Published 2023 Feb 14. doi:10.3390/biomedicines11020557
2022 – Ambrosio RL, Rosselló CA, Casares D, Palmieri G, Anastasio A, Escribá PV. The Antimicrobial Peptide 1018-K6 Interacts Distinctly with Eukaryotic and Bacterial Membranes, the Basis of Its Specificity and Bactericidal Activity. Int J Mol Sci. 2022;23(20):12392. Published 2022 Oct 16. doi:10.3390/ijms232012392
2022 – Beteta-Göbel R, Miralles M, Fernández-Díaz J, et al. HCA (2-Hydroxy-Docosahexaenoic Acid) Induces Apoptosis and Endoplasmic Reticulum Stress in Pancreatic Cancer Cells. Int J Mol Sci. 2022;23(17):9902. Published 2022 Aug 31. doi:10.3390/ijms23179902
2022 – Kumudesh Mishra, Mária Péter, Anna Maria Nardiello, Guy Keller, Victoria Llado, Paula Fernandez-Garcia, Ulf D. Kahlert, Dinorah Barasch, Ann Saada, Zsolt Török, Gábor Balogh, Pablo V.Escriba, Stefano Piotto and Or Kakhlon. Cells 2022.11, 578.
2022 – Javier Fernández-Díaz, Roberto Beteta-Göbel, Manuel Torres, Joan Cabot, Paula Fernández-García, Victoria Lladó, Pablo V.Escribá and Xavier Busquets Frontiers 2022. V12, Art 782525.
2021 – Manuel Torres, Sebastià Parets, Javier Fernández-Díaz, Roberto Beteta-Göbel, Raquel Rodríguez-Lorca, Ramón Román, Victoria Lladó, Catalina A.Rosselló, Paula Fernández-García and Pablo V. Escribá.Membranes 2021.11, 919.
2021 – Garth L. Nicolson, Gonzalo Ferreira de Mattos, Michael Ash, Robert Settineri and Pablo V.Escribá Membranes 2021. 11, 994.
2021 – Roberto Beteta-Göbel, Javier Fernández-Díaz, Laura Arbona-González, Raquel Rodríguez-Lorca, Manuel Torres, Xavier Busquets, Paula Fernández-García, Pablo V.Escribá and Victoria Lladó. Cancers 2021, 13, 4290.
2020 – Hanson, D. Neuro-Oncology, 22(Suppl 3), iii304.
2020 – Torres, M., Rosselló, C. A., Fernández-García, P., Lladó, V., Kakhlon, O., & Escribá, P. V. International Journal of Molecular Sciences, 21(7), 2322.
2020 – Parets, S., Irigoyen, Á., Ordinas, M., Cabot, J., Miralles, M., Arbona, L., Péter, M., Balogh, G., Fernández-García, P., Busquets, X. and Lladó, V. Frontiers in Cell and Developmental Biology, 8, 164.
2019 – Massalha, W., Markovits, M., Pichinuk, E., Feinstein-Rotkopf, Y., Tarshish, M., Mishra, K., Llado, V., Weil, M., Escriba, P.V. and Kakhlon, O. Bioscience reports, 39(1).
2019 – Paula Fernández-García, Catalina A. Rosselló, Raquel Rodríguez-Lorca, Roberto Beteta-Göbel, Javier Fernández-Díaz, Victoria Lladó, Xavier Busquets and Pablo V. Escribá. Cancers 2019, 11, 88
2017 – Ugidos, I. F., Pérez-Rodríguez, D., & Fernández-López, A. Neural regeneration research, 12(8), 1273.
2017 – Ugidos, I.F., Santos-Galdiano, M., Pérez-Rodríguez, D., Anuncibay-Soto, B., Font-Belmonte, E., López, D.J., Ibarguren, M., Busquets, X. and Fernández-López, A. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1859(9), 1648-1656.
2017 – Mohaibes, R.J., Fiol-deRoque, M.A., Torres, M., Ordinas, M., López, D.J., Castro, J.A., Escribá, P.V. and Busquets, X. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1859(9), 1596-1603.
2017 – Casas, J., Ibarguren, M., Álvarez, R., Terés, S., Lladó, V., Piotto, S.P., Concilio, S., Busquets, X., López, D.J. and Escriba, P.V. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1859(9), 1526-1535.
2017 – Noguera-Salvà, M.A., Guardiola-Serrano, F., Martin, M.L., Marcilla-Etxenike, A., Bergo, M.O., Busquets, X. and Escribá, P.V. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1859(9), 1536-1547.
2015 – Álvarez, R., López, D.J., Casas, J., Lladó, V., Higuera, M., Nagy, T., Barceló, M., Busquets, X. and Escribá, P.V. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1851(11), 1511-1520.
2016 – Manuel Torres et al. Update on Dementia. Chapter 7. Dr. Davide Moretti (Ed.) Brain Lipids in the Pathophysiology and Treatment of Alzheimer’s Disease. InTech, DOI: 10.5772/64757
2017 – Escriba PV. Membrane-lipid therapy: A historical perspective of membrane-targeted therapies – From lipid bilayer structure to the pathophysiological regulation of cells.Biochim Biophys Acta Biomembr. 2017 Sep;1859(9 Pt B):1493-1506. doi: 10.1016
2015 – Escribá PV, Busquets X, Inokuchi JI, Balogh G, Török Z, Horváth I, Harwood JL, Vígh L.Membrane lipid therapy: Modulation of the cell membrane composition and structure as a molecular base for drug discovery and new disease treatment. . Prog Lipid Res. 2015 May 9;59:38-53. Review.
2015 – Torres M, Marcilla-Etxenike A, Fiol-deRoque MA, Escribá PV, Busquets X. Apoptosis. 2015 May; 20(5):712-24
2015 – G. Avila-Martin, I. Galan-Arriero, A. Ferrer-Donato, X. Busquets, J. Gomez-Soriano, P.V. Escribá, J. Taylor. Oral 2-hydroxyoleic acid inhibits reflex hypersensitivity and open-field-induced anxiety after spared nerve injury. Eur J Pain. 2015 Jan;19(1):111-22.
2014 – Fernández R, Lage S, Abad-García B, Barceló-Coblijn G, Terés S, López DH, Guardiola-Serrano F, Martín ML, Escribá PV, Fernández JA. Analysis of the lipidome of xenografts using MALDI-IMS and UHPLC-ESI-QTOF. J Am Soc Mass Spectrom. 2014 Jul;25(7):1237-46
2014 – Escribá, P.V. and Nicolson, G.L. Biochimica et biophysica acta, 1838(6), 1449.
2014 – Lladó, V., López, D.J., Ibarguren, M., Alonso, M., Soriano, J.B., Escribá, P.V. and Busquets, X. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1838(6), 1619-1627.
Do you want to know how melitherapy works? We have made an animation video and a series of infographics with clear illustrations and straightforward explanations of this fascinating science.
Feel free to download and share these educational materials, or even republish them — without alteration and with appropriate credit to Laminar Pharma, please!