Products
Thanks to our innovative know-how, we design novel synthetic fatty acid molecules that target the cell membrane to modulate specific signaling pathways. This structure-function based drug design is the foundation of a prolific discovery engine that has allowed us to develop a dynamic and promising pipeline of products — proposing alternative therapeutic strategies for a number of important conditions such as cancer, central nervous system diseases or metabolic disorders.
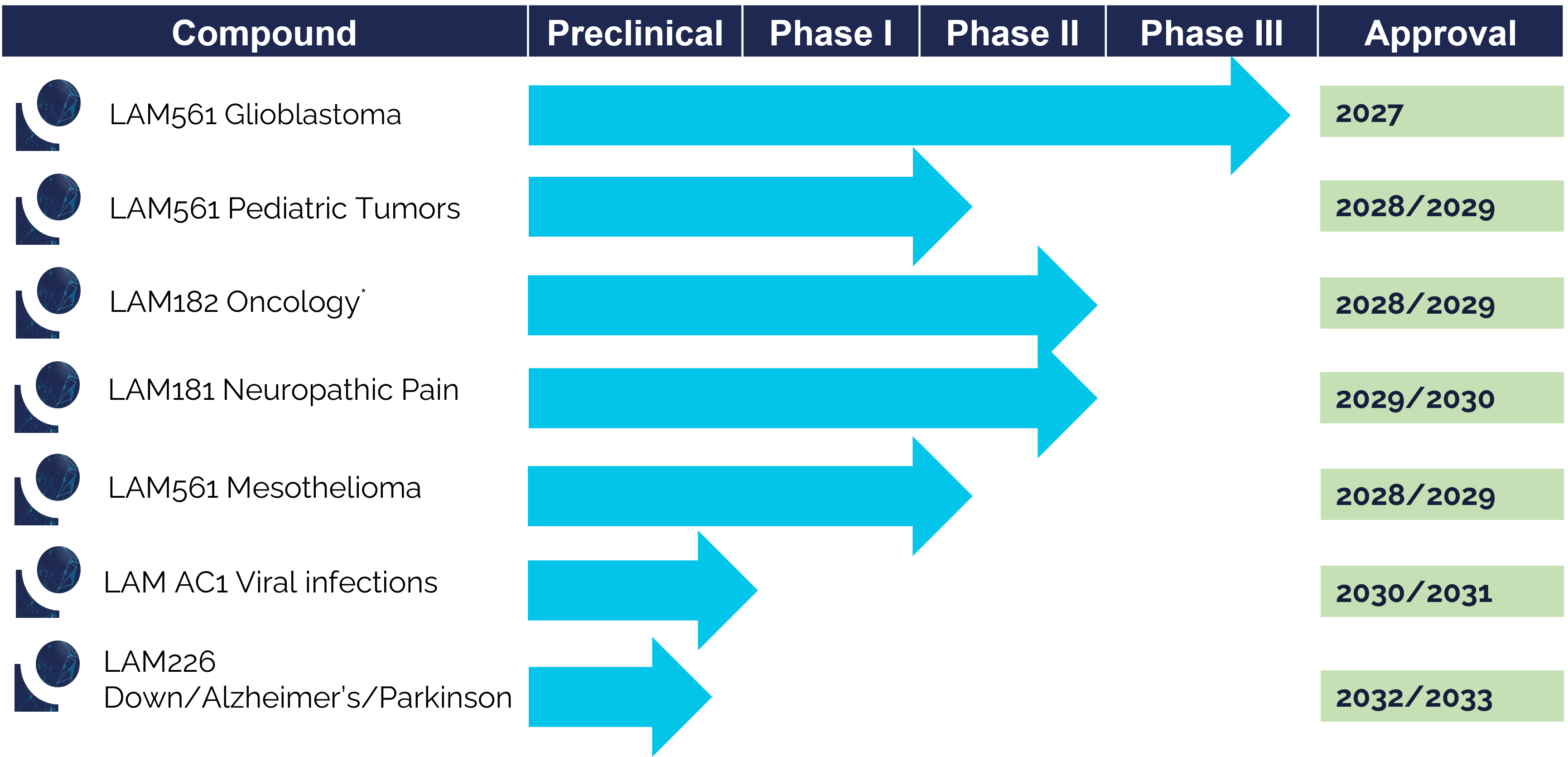
LAM561 (2-hydroxyoleic acid) is a synthetic derivative of oleic acid which can be taken orally and that could be able to reach cells in the brain by crossing the Blood Brain Barrier. This drug alters the composition of the plasma membrane in cancer cells, reducing the activity of membrane-associated signaling proteins that are known to promote tumor growth. Administering LAM561 shows promising results in the treatment of aggressive brain tumors, glioblastomas.
LAM226 is a modified natural fatty acid (DHA derivative) which acts selectively on neurons, due to the specific omega-3 transporters present in neuronal membranes. In studies with mice models of Alzheimer’s disease, this drug has demonstrated recovery of cognitive abilities and recovery of healthy brain biomarkers.
LAM204 is a polyunsaturated fatty acid derivative which has shown a remarkable anti-inflammatory effect in laboratory studies with animal models. Its efficacy is similar to that of steroid compounds, however its non-steroid structure suggests a lower toxicity, so this aspect has been initially assessed in preliminary studies with zebra fish, flies and mice. A collaboration with an external research group (at the University of Leon, Spain) has taken place to explore the potential effect of LAM2014 in an animal model of ischemic lesion following brain stroke, with promising results.
The versatility of the melitherapy approach, targeting membrane lipids of different cell types depending on the pathology, allows us to continuously investigate the therapeutic applications of new compounds.
LAM30171 is the latest candidate, a new fatty acid derivative currently being investigated as a potential treatment for cancer and metabolic disorders.
We collaborate with bigger pharmaceutical companies with relevant structure to distribute and sell medicines in wide continental areas globally. Companies interested in receiving information about Laminar’s licensing opportunities can contact us using the form below: